how many valence electrons does alkaline earth metals have|Group 2 Elements: The Alkaline Earth Metals : Pilipinas All the alkaline earth metals have two electrons in their valence shell, so the energetically preferred state of achieving a filled electron shell is to lose two electrons to form doubly charged positive ions. Tingnan ang higit pa Sambong (scientific name: Blumea balsamifera) is an amazing medicinal plant. Coming from the family of Compositae, it goes by several names locally. It is known in the Visayas as bukadkad and as subsob in Ilocos. The plant is a strongly aromatic herb that grows tall and erect. Its height ranges from 1.5 to 3 meters, with stems that grow for up .
PH0 · What Are the Properties of the Alkaline Earth Metals?
PH1 · The Valence Orbitals of the Alkaline
PH2 · How many valence electrons do alkaline earth metals have
PH3 · Group 2 Elements: The Alkaline Earth Metals
PH4 · Chapter 20.4: The Alkaline Earth Metals (Group 2)
PH5 · Alkaline earth metals: their chemical characteristics
PH6 · Alkaline earth metal
PH7 · Alkaline Earth Metals: Definition & Location in the Periodic Table
PH8 · Alkaline Earth Metals
PH9 · 6.10: Alkaline Earth Metals
PH10 · 20.5: The Alkaline Earth Metals (Group 2)
PH11 · 12.3: Group II
All authorized signatories must have Class, 3 Digital Signature Certificate. India most of the e-Filling is done through Chartered Accountants (CA’s), Company Secretary (CS’s), CWA and Tax Consultants and Advocates Class 3 Digital Signature Certificate for e-Tendering, e-Procurement & e-Bidding . . Features of digital signature cost .
how many valence electrons does alkaline earth metals have*******All the alkaline earth metals have two electrons in their valence shell, so the energetically preferred state of achieving a filled electron shell is to lose two electrons to form doubly charged positive ions. Tingnan ang higit paThe alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar . Tingnan ang higit pa
ChemicalAs with other groups, the members of this family show patterns in their electronic configuration, . Tingnan ang higit paMost beryllium is extracted from beryllium hydroxide. One production method is sintering, done by mixing beryl, sodium fluorosilicate, and soda at high temperatures . Tingnan ang higit paReaction with halogensCa + Cl2 → CaCl2Anhydrous calcium chloride is a hygroscopic substance that is used as a desiccant. . Tingnan ang higit pa
how many valence electrons does alkaline earth metals haveEtymologyThe alkaline earth metals are named after their oxides, the alkaline earths, whose old-fashioned names were beryllia, magnesia Tingnan ang higit paBeryllium occurs in the Earth's crust at a concentration of two to six parts per million (ppm), much of which is in soils, where it has a concentration of six ppm. Beryllium is one of . Tingnan ang higit paBeryllium is used mainly in military applications, but non-military uses exist. In electronics, beryllium is used as a p-type dopant in . Tingnan ang higit paIn contrast, the alkaline earth metals generally have little or no tendency to accept an additional electron because their ns valence orbitals are already full; an added .
Alkaline earth metals have two valence electrons. They have low ionization energy, low electron affinity, and low electronegativity. They are highly reactive and often form divalent cations. They are good . Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The alkaline earth metals have a greater . Although many characteristics are common throughout the group, the heavier metals such as Ca, Sr, Ba, and Ra are almost as reactive as the Group 1 Alkali . The alkaline-earth (Ae) metals of group 2 of the periodic table of elements traditionally belong to the class of main-group atoms and their valence electrons .
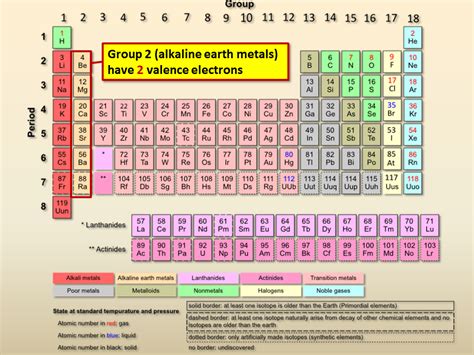
The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Atoms of each of these elements have two electrons in the outer electron shell. . This may be attributed to the general valence electron configuration ns 2 for the alkaline earths, which involves two electrons per metal atom in metallic bonding (instead of just one as in an alkali metal).How many electrons are in the outer shell of the alkaline earth elements? Are the alkaline earth elements more or less reactive than the alkali metals? Explain your answer.how many valence electrons does alkaline earth metals have Group 2 Elements: The Alkaline Earth Metals Alkaline earth metals have 2 valence electrons. This makes them reactive, but less so than alkali metals, which have 1 valence electron.

Calcium is the 20th element in the periodic table. It is a group 2 metal, also known as an alkaline-earth metal, and no populated d-orbital electrons. Calcium is the fifth most abundant element by mass (3.4%) in both the Earth's crust and in seawater. All living organisms (in fact, even dead ones) have and need calcium for survival. Calcium . The alkaline earth metals have 2 valence electrons. They are located in Group 2 of the periodic table and include elements such as beryllium, magnesium, calcium, strontium, barium, and radium. The number of valence electrons corresponds to the group number, so the alkaline earth metals have 2 valence electrons. Learn more about .
The presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: For a main group element, a valence electron can only be in the outermost electron shell. An atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be chemically .
Alkaline earth metals have two valence electrons. They are highly reactive and are not found naturally in their elemental state. The two valence electrons are easily removed to form divalent cations. . and low electronegativity. Alkaline earth metals have an oxidation state of +2 and an electronic structure in the S subshell occupied by 2 .
Although many of these properties are similar to those of the alkali metals (Table \(\PageIndex{1}\)), certain key differences are attributable to the differences in the valence electron configurations of the two groups (ns 2 for the alkaline earth metals versus ns 1 for the alkali metals).The alkaline earth metals have how many valence electrons? How many valence electrons do the alkaline-earth elements have? A. 1 B. 2 C. 7 D. 8; Of Ba, F, Ne, and K, which one is an alkaline earth metal? How many valence electrons does it have? What is the valence electron configuration for alkaline earth metals?
Alkaline Earth Metals. Group 2 elements are referred to as “alkaline earth” metals (tan column below).The name “alkaline” comes from the fact that compounds of these elements form basic (pH greater than 7) or alkaline solutions when dissolved in water.If the Group 1 elements all have one \(s\) electron in their outer orbital, we can predict that the Group . The alkaline earth metals have 2 valence electrons. Explanation: Alkaline earth metals are the family of metals that belong to group (column) number 2 in the periodic table. You can tell the number of valence electrons of the representative elements by the number of column: the ones digit is the number of valence electrons. .If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. All alkali metals, alkaline earth metals, and halogens have a common valence electron configuration: alkali metals have 1 valence electron, alkaline earth metals have 2 valence electrons, and . Alkali Metals: Group 1 (IA) - one valence electron; Alkaline Earth Metals: Group 2 (IIA) - two valence electrons; Transition Metals: Groups 3-12 - two valence electrons; Boron Group or Earth .
How Many Valence Electrons to Alkaline Earth Metals Have? Valence electrons refer to the number of electrons found in the outermost shell of an atom. Alkaline earth metals are characterized by .Alkaline earth metals have two valence electron, whereas alkali metals have just one. This makes the former less reactive than the latter [11]. Interesting Facts. The alkaline earth metals are the most reactive element family after the alkali metals [5]. The first and last element of Group 2, Be and Ra, are both toxic to living organisms [1].
All alkali metals ( belongs to group 1 of periodic table) have 1 valence electron. Valence electron are those electron that are present in the outermost orbit of the atom. Alkali metal present in group 1 of periodic table: Hydrogen (H), Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs),and Francium (Fr). The alkali metals or .We would like to show you a description here but the site won’t allow us. First and second ionization energies for the alkaline earths (corresponding to removal of the first and second valence electrons) are relatively small, but the disruption of an octet by removal of a third electron is far more difficult. Like the alkali metals, the alkaline-earth atoms lose electrons easily, and so they are good reducing agents.
The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Atoms of each of these elements have two electrons in the outer electron shell. Take a look at the elements in this group and their common properties: List of the Alkaline Earth Metals. There are six alkaline earths.
Hotels Near Moscone Center Reviews: There are 3,52,305 reviews on Tripadvisor for Hotels nearby: Hotels Near Moscone Center Photos: There are 1,40,402 photos on Tripadvisor for Hotels nearby Nearest accommodation: 0.12 km: Frequently Asked Questions about hotels near Moscone Center.
how many valence electrons does alkaline earth metals have|Group 2 Elements: The Alkaline Earth Metals